
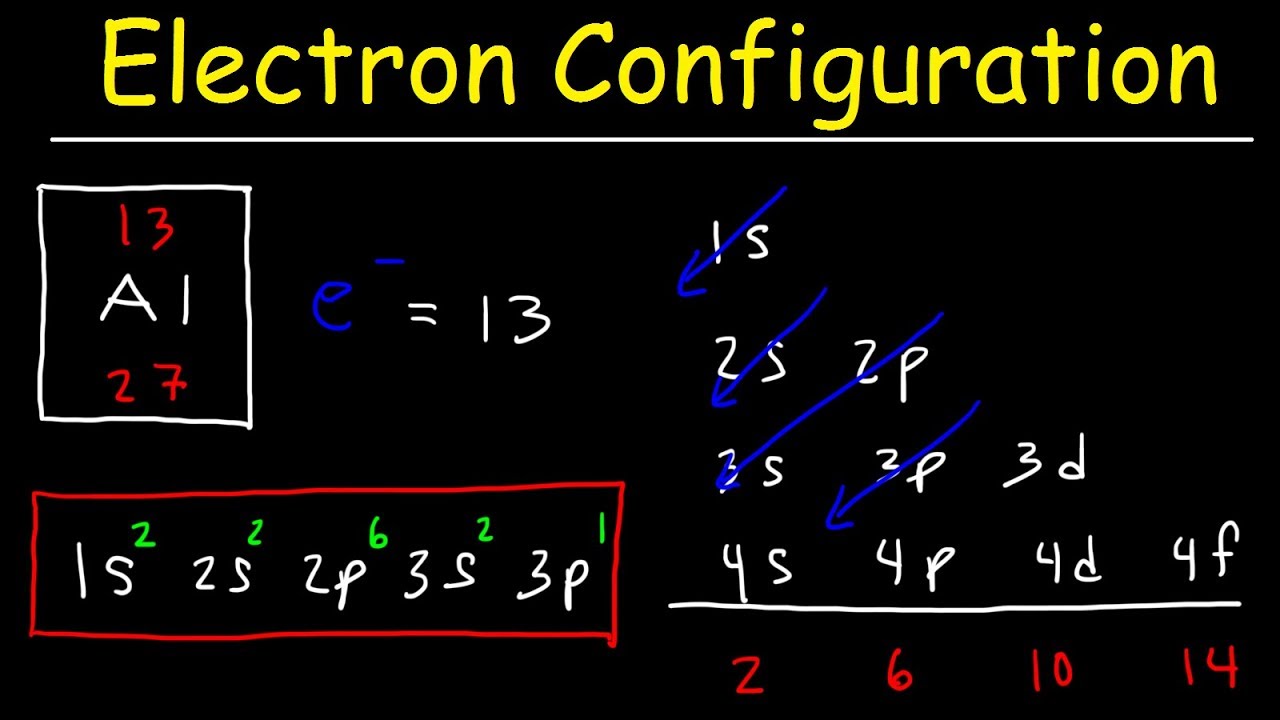
On the other hand, the d electrons in transition metals are not de-localized in metallic bonding of transition metals (to the best of my knowledge). In the below periodic table you can see the trend of. This arrangement is emphasized in Figure, which shows in periodic-table form the electron configuration of the last subshell to be filled by the Aufbau principle. On one hand, most of the oxidation states for transition metals have been observed, up to the point where they would have lost all of those d electrons. Now we can understand why the periodic table has the arrangement it hasthe arrangement puts elements whose atoms have the same number of valence electrons in the same group. Many sources will refer to all of the electrons above the configuration of the prior noble gas to be valence electrons. Valence Electrons and the Periodic Table 408,500 views This chemistry video tutorial provides a basic introduction into valence electrons and the periodic table. Transition metals in the highest d sublevel (but not in the highest primary energy level) can provide valence. This will give you the number of electrons in the highest energy level for transition metals. The number of valence electrons in an atom is reflected by its position in the periodic table. An atom with one, two, or three valence electrons usually reacts by losing these electrons to acquire the electron configuration of the noble gas next below it. It turns out to be a d-block element that is not filling the d-block. Answer: Electrons in the outermost shells are called valence electrons. This is a key first step for drawing Lewis dot s. It has 18 in its highest energy level, level 4, but only the 10 4d electrons are considered to be valance electons.Īnd Lawrencium which has 7s2 (5f14) xp1, or 3 valence electrons, putting 1 electron in the xp sublevel rather than in the (x-1)d sublevel. An explanation and practice for finding the number of valence electrons for elements on the periodic table. Palladium which prefers to fill the x-1d sublevel and not put any in the xs sublevel. Since the Aufbau priniciple fails to account for the fact that some elements will be more stable with incomplete valence xs sublevels and additional electrons in the (x-1)d sublevel, you just need to memorize the transition metals that are more stable with 1 valence s electron:Ĭhromium, Possibly Nickel, Copper, Niobium, Molybdenum, Ruthenium, Rhodium, Silver, Platinum and Gold,
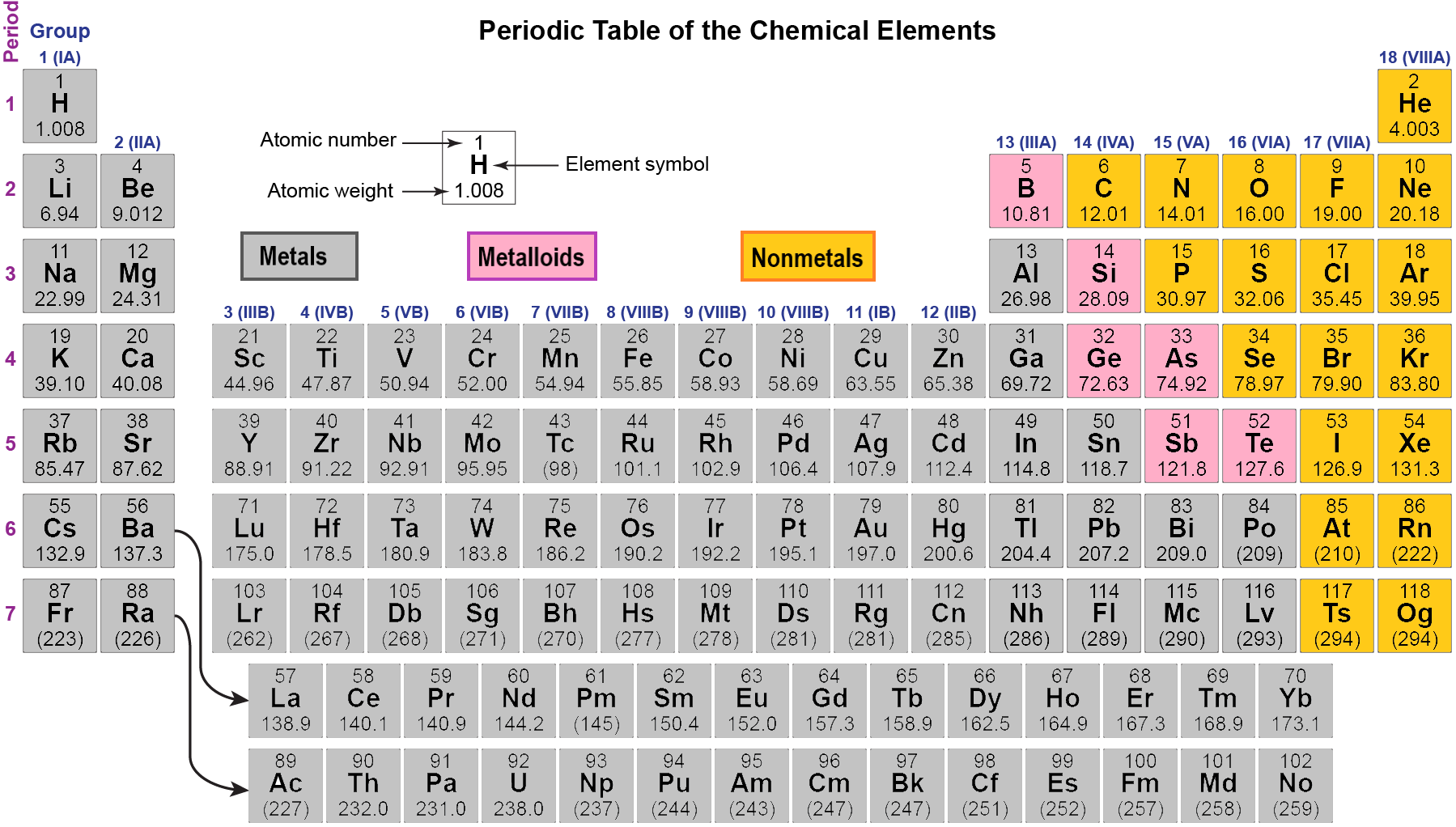
Based on the Aufbau principle, they would all be filling the (x-1)d sublevel, so they would ALL have 2 valence electons in the xs sublevel.


 0 kommentar(er)
0 kommentar(er)
